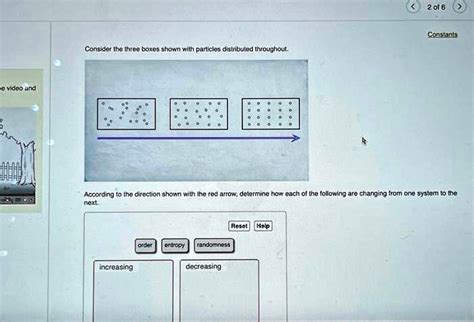
consider the three boxes shown with particles distributed throughout. Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level . $14.95
0 · VIDEO solution: 2 of 6 Constants Consider the three boxes
1 · U4 Hw3: density Flashcards
2 · Solved Select Play to watch the video on the second law of
3 · Solved Consider the three boxes shown with particles
4 · SOLVED: Consider the three boxes shown with particles
5 · Figure 1 B FIGURE 1 Worksheet Density A B Comparison of
6 · Entropy and a box of air
7 · Consider the three boxes shown with particles distributed
Using the Box and Pan Brake. I think it’s fairly obvious how a bending brake works. Put metal in, clamp, pull lever. No need for elaborate text when a handful of pictures will do: Clamp down for the first bend. Pull the lever. Reposition for the second bend. Note the relief notch. Pull the lever. You can see the test piece on the right.
Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn .
Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level . Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A .
As we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules.
2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the .Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table . Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the .Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how .
Consider the three boxes shown with particles distributed throughout. Each box from left to right has 2 0 particles inside. The particles in the first box are scattered around with no apparent .Question: Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next.
Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn solution you can count on.
VIDEO solution: 2 of 6 Constants Consider the three boxes
U4 Hw3: density Flashcards
under cabinet two outlet junction box
Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level of order, as the particles are evenly distributed. Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A and B compare? *Mass A > Mass B *Mass A < Mass B *Mass A = Mass BAs we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules.
2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next: Entropy Randomness increasing decreasingEach dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table below, show how the masses, volumes, and densities of A and B compare by adding the symbol <, >, or = to the statement in the second column. Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the direction of the red arrow.
Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next. Your solution’s ready to go!Consider the three boxes shown with particles distributed throughout. Each box from left to right has 2 0 particles inside. The particles in the first box are scattered around with no apparent pattern. The particles in the second box are close to forming a grid that is four particles high and five particles long.
Question: Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next.
Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn solution you can count on. Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level of order, as the particles are evenly distributed. Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A and B compare? *Mass A > Mass B *Mass A < Mass B *Mass A = Mass B
As we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules. 2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next: Entropy Randomness increasing decreasingEach dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table below, show how the masses, volumes, and densities of A and B compare by adding the symbol <, >, or = to the statement in the second column. Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the direction of the red arrow.
Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next. Your solution’s ready to go!
underground junction box cable
Solved Select Play to watch the video on the second law of
Tools: New and gently used hand and power tools can also be found in great .
consider the three boxes shown with particles distributed throughout.|Consider the three boxes shown with particles distributed